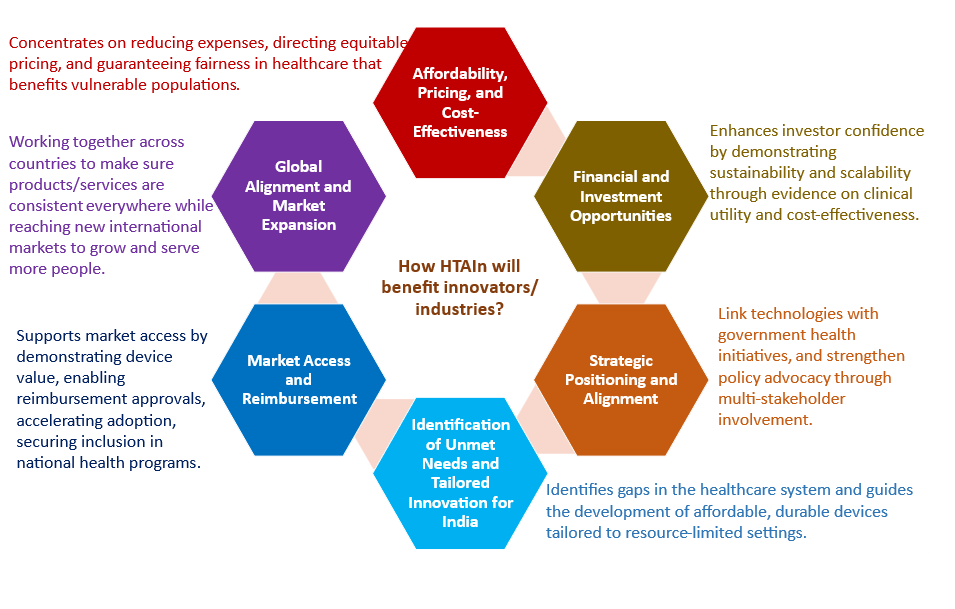
In India’s rapidly evolving healthcare system, HTA plays a pivotal role in aligning technologies with the needs of patients, providers, and policymakers while helping innovators gain a competitive edge. It serves as a vital tool for validating technologies, securing market acceptance, and driving adoption in a cost-sensitive, equity-focused landscape. Beyond supporting innovators, HTA contributes to improving public health outcomes by facilitating the integration of innovative technologies in healthcare delivery.
Innovators are encouraged to log in to the HTAIn topic submission Portal to initiate the HTA process for their technology
Innovators are requested to provide the following evidence to process their products for HTA.
| Evidence | Description | Types of Documents |
|---|---|---|
| Regulatory and Compliance Documentation | Approvals from relevant regulatory bodies,and adherence to national/international standards for safety and quality | CDSCO Certificate |
| Product Details | Device specifications, functionality, usability, operational requirements like infrastructure/personnel needs, maintenance, training, and lifecycle cost. | Innovation Website |
| Clinical Evidence | Clinical trial data demonstrating the safety, efficacy, and effectiveness of the product. Real-world evidence or observational studies, if applicable. Comparative studies showing advantages over existing alternatives. | Systematic Review of clinical evidence (if any). Preferable published evidence in a peer-reviewed journal. In case of unavailability of published evidence information in the form of a MANUSCRIPT |
| Stakeholder Feedback (If any) | Input from healthcare providers, payers, and end-users.Results from pilot studies or field tests, if available. |